You are here
Content
Angiodiversity and Organ Function
Angiodiversity
The microvasculature displays remarkable structural and functional diversity. Angiodiversity occurs along the segments of the vascular tree and in the microvascular beds of different organs ranging from continuous, barrier-forming endothelium to discontinuous, fenestrated endothelia. Endothelial heterogeneity mediates organ-specific differentiation of the microvascular niche comprising various other cell populations besides endothelial cells such as vascular wall cells (pericytes), parenchymal and stromal cells, but also stem cells. Organ-specific differentiation of the microvascular niche is induced by so-called angiokines. Angiokines are cytokines that are produced by endothelial cells and display an organ-specific pattern of secretion.
Hepatic Angiodiversity
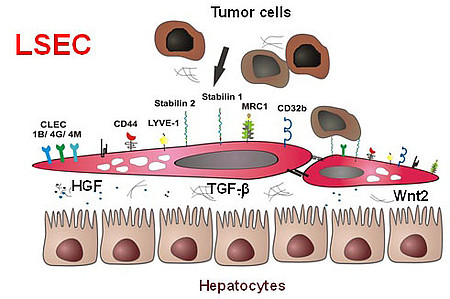
The microvascular bed of the liver represents a prime example of the organ-specific angiovidversity of the microvascular niche. Here, the organ-specific microvascular endothelial cells are called liver sinusoidal endothelial cells (LSEC). LSEC differ from normal endothelial cells by their special morphology including lack of a basement membrane. Pathological changes of these highly specialized, discontinuous liver sinusoids contribute to severe liver diseases such as alcoholic steatohepatitis and liver cirrhosis. During hepatocarcinogenesis and metastasis to the liver, LSEC trans-differentiate towards a capillary phenotype (capillarization). Normal LSEC display a high capacity of endocytosis for clearance of noxious factors from the peripheral blood. To this end they have a complex cytoskeleton with sieve plates and fenestrations and they express a special set of endocytic receptors. LSEC are also involved in regulating portal blood pressure.
Hepatic endothelial scavenger receptors
In the recent years, our group has been able to identify several novel sinusoidal endothelial proteins using classical biochemical methods as well as novel molecular approaches including gene expression profiling. Stabilin1 (Stab1) and Stabilin2 (Stab2) were first identified as novel marker proteins of discontinuous sinusoidal endothelial cells; further bioinformational analysis was used to classify them as endocytic scavenger receptors. In addition, we could show that the stabilins considerably contribute to the clearance function of LSEC. As ligands for the stabilins, we identified a wide range of molecules, i.e. hyaluronic acid, AGE-modified proteins, the extracellular matrix protein SPARC and others such as chitiniase-like proteins (SICLP), cytokines (GDF15), and hormones (placental lactogen).
Endothelial Clearance in the liver: local and systemic effects
In order to analyze the scavenger functions of LSEC in more detail, Stab1- and Stab2-deficient mice were generated. While Stab1 and Stab2 single knockout mice were grossly normal, mice deficient for both stabilins dye early and suffer from severe hepatic and renal fibrosis. In complicated animal and transplantation experiments, we could show that glomerulofibrotic nephropathy of stab1/2-double deficient mice is caused by impaired clearance of noxious blood substances from the peripheral circulation by LSEC. Thus, this seminal work by our group showed that LSEC-mediated clearance of noxious factors from the circulation by Stab1/2 exerts systemic effects, i.e. in protecting the integrity of the kidney.
Gata4: a master regulator of angiocrine signaling in the liver
While the transcriptional programs regulating arterial, venous and lymphatic specification have been characterized in substantial molecular detail in recent years, a master regulatory transcription factor such as Prox-1 for lymphatic EC could not be identified for blood microvascular EC. Therefore, it came as a major break¬through that the combination of the transcription factors etv2, erg, and fli was sufficient to induce full continuous blood vascular endothelial reprogramming in amnion cells. In contrast, the molecular regulators driving organ-specific differentiation of microvascular EC have not yet been comprehensively elucidated. This dictum also applies to hepatic angiodiversity. While Id1, liver X receptor (LXR)-α, endothelial Notch signaling, KLF2, and Erg have been shown to contribute to the molecular differentiation program of LSEC, a molecular master regulator for LSEC specification and differentiation has hitherto been elusive.
Fortunately, we have now been able to identify the transcription factor Gata4 as molecular master regulator for LSEC differentiation. Gata4 also controls down-stream angiocrine functions of LSEC on liver homeostasis and metabolic maturation mediated by Bmp2, Wnt, and Rspo3 signaling. Using newly generated LSEC-specific cre-driver lines (stab2-iCre), LSEC-restricted deletion of Gata4 was embryonic lethal and caused transformation of discontinuous liver sinusoids into continuous capillaries (sinusoidal capillarization).
This switch from discontinuous LSEC to continuous EC also impaired immigration of hematopoietic progenitor cells into the liver resulting in lethal anemia. Preliminary results show that LSEC-specific Gata4 deficiency in adult mice causes hepatopathy and liver fibrosis also accompanied by sinusoidal capillarization. Further experiments have recently shown that Gata4 is not the only transcriptional regulator important for LSEC development and function. Activation of Notch also impairs liver growth and metabolic maturation and alters organotropic metastatic processes.
Hepatic angiocrine signaling controls liver growth, metabolic maturation and iron metabolism
Among the Gata4-dependent LSEC-specific genes, we identified Bmp2 and the Wnt-related factor Rpso3 as novel LSEC-specific angiokines. We could show that angiocrine Bmp2 signaling within the hepatic vascular niche represents a constitutive pathway indispensable for iron homeostasis in vivo by controlling hepcidin. After we had identified wnt2 as an LSEC-associated angiokine with autocrine growth effects, we have now expanded on these results showing that angiocrine wnt signaling in the liver controls liver development and metabolic maturation.
In our group, we focus on two lines of experiments to further investigate the basic mechanisms and functions of hepatic angiodiversity and liver function:
- Identify and functionally characterize the mechanisms and especially the transcriptional regulators that regulate and control LSEC differentiation during development and in the adult organism in health and disease
- Define the role of endothelial heterogeneity and of the organ-specific cellular and molecular interactions within the hepatic vascular niche during hepatotropic metastasis and hepatocarcinogenesis
Project-related publications
- Leibing T*, Géraud C*, Augustin I, Boutros M, Augustin HG, Okun JG, Langhans CD, Zierow J, Wohlfeil SA, Olsavszky V, Schledzewski K, Goerdt S, Koch PS: Angiocrine Wnt signaling controls liver growth and metabolic maturation in mice. Hepatology. 2017 Oct 23. doi: 10.1002/hep.29613. [Epub ahead of print]
- Géraud C*, Koch PS*, Zierow J*, Klapproth K, Busch K, Olsavszky V, Leibing T, Demory A, Ulbrich F, Diett M, Singh S, Sticht C, Breitkopf-Heinlein K, Richter K, Karppinen SM, Pihlajaniemi T, Arnold B, Rodewald HR, Augustin H, Schledzewski K, Goerdt S: Gata4-dependent organ-specific endothelial differentiation controls liver development and embryonic hematopopiesis. J Clin Invest, 127: 1099-114, 2017
- Olsavszky V, Ulbrich F, Singh S, Diett M, Sticht C, Schmid CD, Zierow J, Wohlfeil SA, Schledzewski K, Dooley S, Gaitantzi H, Breitkopf-Heinlein K, Géraud C, Goerdt S, Koch PS. GATA4 and LMO3 balance angiocrine signaling and autocrine inflammatory activation by BMP2 in liver sinusoidal endothelial cells. Gene, 27:491-499, 2017.
- Koch PS*, Olsavszky V*, Ulbrich F, Sticht C, Demory A, Leibing T, Henzler T, Meyer M, Zierow J, Schneider S, Breitkopf-Heinlein K, Gaitantzi H, Spencer-Dene B, Arnold B, Klapproth K, Schledzewski K, Goerdt S*, Géraud C*: Angiocrine Bmp2 signaling in murine liver controls normal iron homeostasis. Blood 129: 415-9, 2017 (*equal contribution).
- Runge A, Hu J, Wieland M, Bergeest JP, Mogler C, Neumann A, Géraud C, Arnold B, Rohr K, Komljenovic D, Schirmacher P, Goerdt S, Augustin HG : An inducible hepatocellular carcinoma model for preclinical evaluation of antiangiogenic therapy in adult mice. Cancer Res, 74, 4157-69, 2014
- Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. Felcht M, Luck R, Schering A, Seidel P, Srivastava K, Hu J, Bartol A, Kienast Y, Vettel C, Loos EK, Kutschera S, Bartels S, Appak S, Besemfelder E, Terhardt D, Chavakis E, Wieland T, Klein C, Thomas M, Uemura A, Goerdt S, Augustin HG: J Clin Invest, 122, 1991-2005, 2012.
- Schmieder A, Schledzewski K, Michel J, Schönhaar K, Morias Y, Bosschaerts T, Van den Bossche J, Dorny P, Sauer A, Sticht C, Géraud C, Waibler Z, Beschin A, Goerdt S: The CD20 homolog Ms4a8a integrates pro- and anti-inflammatory signals in novel M2-like macrophages and is expressed in parasite infection. Eur J Immunol, 42, 2971-82, 2012.
- Schmieder A, Schledzewski K, Michel J, Tuckermann JP, Tome L, Sticht C, Gkaniatsou C, Nicolay JP, Demory A, Faulhaber J, Kzhyshkowska J, Géraud C, Goerdt S: Synergistic activation by p38MAPK and glucocorticoid signaling mediates induction of M2-like tumor-associated macrophages expressing the novel CD20 homolog MS4A8A. Int J Cancer, 129, 122-32, 2011.
- Schledzewski K, Géraud C, Arnold B, Wang S, Gröne HJ, Kempf T, Wollert KC, Straub BK, Schirmacher P, Demory A, Schönhaber H, Gratchev A, Dietz L, Thierse HJ, Kzhyshkowska J, Goerdt S: Deficiency of liver sinusoidal scavenger receptors stabilin-1 and -2 in mice causes glomerulofibrotic nephropathy via impaired hepatic clearance of noxious blood factors. J Clin Invest, 121: 703-14, 2011.
- Géraud C*, Schledzewski K*, Demory A, Klein D, Kaus M, Peyre F, Sticht C, Evdokimov K, Lu S, Schmieder A, Goerdt S: Liver sinusoidal endothelium: a microenvironment-dependent differentiation program in rat including the novel junctional protein liver endothelial differentiation-associated protein-1. Hepatology, 52: 313-326, 2010.
Context Column
Contact

Prof. Dr. Sergij Goerdt
Dept. Of Dermatology, Venereology, and Allergy
Research Group "Angiodiversity and Organ Function" together with Dr. Philipp-Sebastian Koch
University Medical Center and Medical Faculty Mannheim
Heidelberg University
Theodor-Kutzer-Ufer 1-3
68167 Mannheim
Phone +49 621 383-2280
sergij.goerdt@umm.de