You are here
Content
Neuropharmacology
The Institute of Psychopharmacology is primarily concerned with addiction research. The focus of our interest is the animal experimental and translational research of alcohol and drug addiction. Since addictive behavior often occurs with other psychiatric disorders, especially anxiety, depression, and ADHD, we also study these comorbidities. We also conduct research on social exclusion and borderline personality disorders.
Five research groups are affiliated to the Institute of Psychopharmacology: Behavioral Pharmacology (Spanagel), Behavioral Genetics (Bilbao), Neuroanatomy (Hansson), Molecular Pharmacology (Sommer), Translational Addiction Research (Sommer, Kiefer).
Based on preclinical findings, we pursue three objectives:
- the development of new behavioral therapies, pharmacological interventions and neuromodulatory approaches in addicted patients (e.g. pharmacological suppression of drug memory reconsolidation)
- to clarify the neurobiological long-term consequences of drug abuse and binge drinking in adolescents
- the identification of risk factors for addictive disorders and development of preventive strategies
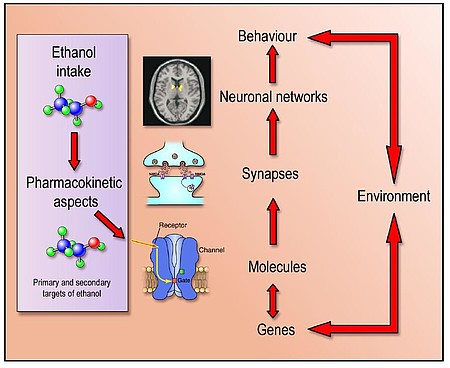
We use a wide range of methods to pursue our goals. In the field of behavioural pharmacology we use a variety of standard models (e.g. Reinstatement Model) and two new animal models for the generation of alcohol-addicted and cocaine-addicted rats respectively. These addiction models are the starting point for characterizing the neuroanatomical and molecular substrates of addictive behavior. Furthermore, these models are used to preclinically test new anti-relapse compounds in cooperation with the pharmaceutical industry.
For this purpose we additionally use in silico methods such as drug repurposing. Furthermore, we investigate gene x environment interactions that contribute to an increased risk of addiction. For this purpose we use different transgenic animal models in interaction with the drug and environmental factors such as stressors. In translational research we use combined animal and human MRI based methods (spectroscopy, fMRI, phMRI, and anatomical MRI approaches with 9.4T and 3T scanners, respectively) as well as converging genomic analyses - candidate genes from genome-wide association studies and differential gene expression analyses are defined and then functionally validated in transgenic rat models. Another important translational approach is our in silico human brain bank where we are integrating –omics derived information (also on a single cell level) for alcohol, opiate, cocaine, cannabis and nicotine use disorders.
Joint and International Research Projects
2019-2012 EU ERA-Net NEURON: Psi-Alc 01EW1908
"Preclinical Phase II Testing of Psilocybin in Alcohol Addiction and Epigenetic and Neuroimaging Studies on the Mode of Action"
2015-2020 BMBF AERIAL 01EE1406C
"Addiction: Early Recogition and Intevention Across the Lifespan (AERIAL: Mechanisms of addiction: social exclusion, risk and resilience prediction, and adapted interventions"
2019-2022 BMBF Target-OXY 031L0190A
"Towards a targeted treatment of alcohol addiction with oxytocin"
2019-2022 BMBF eMed SysMedSUDs Systems Medicine Approach for Substance Use Disorders 01ZX1909A
2018-2022 DFG GRK 2350
"Impact of Adverse Childhood Experiences on Psychosocial and Somatic Conditions Across the Lifespan"
2015-2023 DFG SFB 1158 Pain
"Translational studies in chronicity of pain: Neuroplasticity in corticolimbic dopamine and glutamate pathways"
2019-2022 DFG Sequencing Centers
"Deciphering alcohol addiction associated gene regulation changes on a single cell level"
2019-2023 DFG TRR265 Addiction
"Intensive behavioral monitoring and dynamical state transitions in animal models of addiction"
Selected Publications
- Bernardi RE, Olevska A, Morella I, Fasano S, Santos E, Brambilla R, Spanagel R (2019) The inhibition of RasGRF2, but not RasGRF1, alters cocaine reward in mice. J Neurosci 39(32):6325-6338
- Noori HR, Mervin LH, Bokharaie V, Durmus Ö, Egenrieder L, Fritze S, Reinhardt G, Schabel HH, Staudenmaier S, Logothetis NK, Bender A, Spanagel R (2018) Systemic neurotransmitter responses to clinically approved and experimental neuropsychiatric drugs. Nat Commun 9(1):4699
- Cannella N, Oliveira AMM, Hemstedt TJ, Lissek T, Bading H, Spanagel R (2018) Dnmt3a2 in the nucleus accumbens shell is required for reinstatement of cocaine-seeking. J Neurosci 38(34):7516-7528
- Noori HR, Schöttler J, Ercsey-Ravasz M, Cosa-Linan A, Varga M, Toroczkai Z, Spanagel R (2017) A multiscale cerebral neurochemical connectome of the rat brain. PLoS Biol 15(7):e2002612
- Hirth N, Meinhardt MW, Noori HR, Salgado H, Torres-Ramirez O, Broccoli L, Vengeliene V, Roßmanith M, Perreau-Lenz S, Köhr G, Sommer WH, Spanagel R, Hansson AC (2016) Convergent evidence from alcohol-dependent humans and rats for a hyperdopaminergic state in protracted abstinence. Proc Natl Acad Sci U S A 113(11):3024-9
- Schneider M, Kasanetz F, Monory K, Leweke FM, Schreckenberger M, Lutz B, Reggio PH, Manzoni OJ, Spanagel R (2015) Enhanced functional activity of the cannabinoid type-1 receptor mediates adolescent behavior. J Neurosci 35(41):13975-88
- Vengeliene V, Leonardi-Essmann F, Sommer WH, Spanagel R (2010) Glycine transporter-1 blockade leads to persistently reduced relapse-like alcohol drinking in rats. Biol Psychiatry 68(8):704-11.
- Engblom D, Bilbao A, Sanchis-Segura C, Dahan L, Perreau-Lenz S, Balland B, Parkitna JR, Luján R, Halbout B, Mameli M, Parlato R, Sprengel R, Lüscher C, Schütz G, Spanagel R (2008) Glutamate receptors on dopamine neurons control the persistence of cocaine seeking. Neuron 59(3):497-508.
- Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M, Holzberg D, Soyka M, Schreiber S, Matsuda F, Lathrop M, Schumann G, Albrecht U (2005) The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med 11(1):35-42.
- Sillaber I, Rammes G, Zimmermann S, Mahal B, Zieglgänsberger W, Wurst W, Holsboer F, Spanagel R (2002) Enhanced and delayed stress-induced alcohol drinking in mice lacking functional CRH1 receptors. Science 296(5569):931-3.
Context Column
Kontakt

Prof. Dr. Rainer Spanagel
Institute for
Psychopharmacology
Head
Zentralinstitut für Seelische Gesundheit (ZI)
J5
68159 Mannheim
Deutschland
Phone +49 621 1703-6251
rainer.spanagel@zi-mannheim.de
