You are here
Content
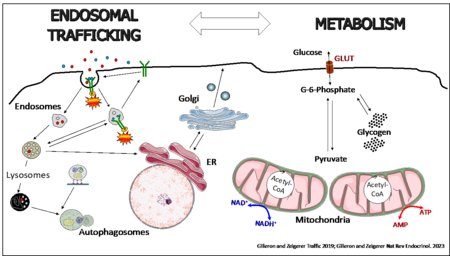
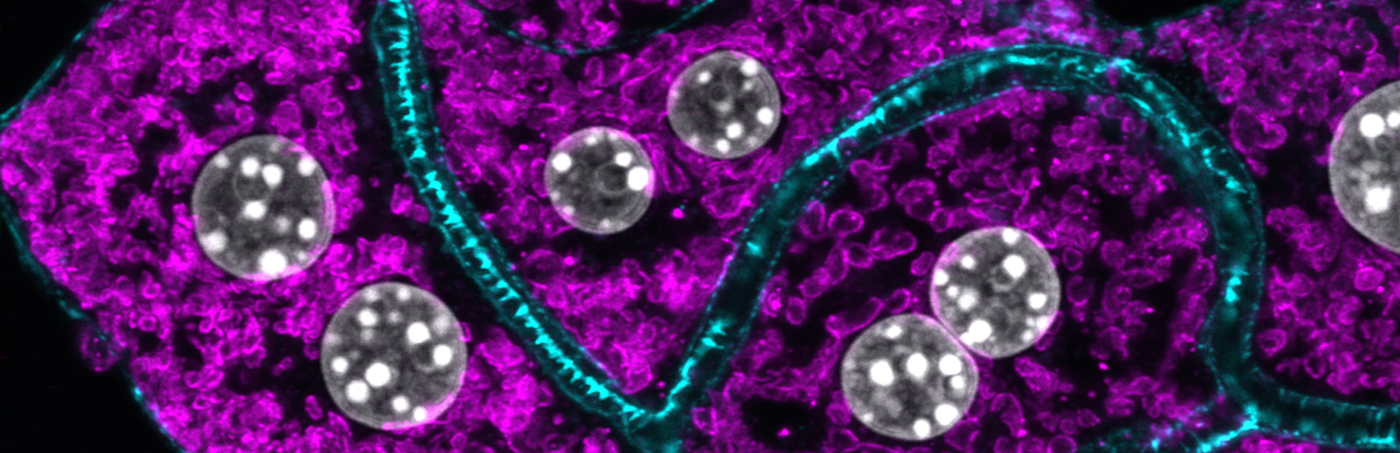
The liver maintains glucose and lipid homeostasis by adapting its metabolic activity to the energy needs of the body. Communication between hepatocytes and the extracellular environment mediated via endocytosis is key to such homeostasis. Endocytosis is an essential mechanism for the uptake and signal transduction of growth factors and hormones but also for regulating metabolic activities, by changing the expression of hormone receptors and nutrient transporters on the cell surface of metabolically active tissue. Thus, we are interested how intracellular endo-lysosomal transport processes affect liver lipid and glucose metabolism with the focus on metabolic diseases, such as type-2 diabetes and obesity. On the other hand, we investigate the influence of extracellular nutritional cues on intracellular membrane transport.
To address these research areas, we use primary mouse and human hepatocyte 3D cultures, where hepatocytes regain cell polarity and liver-specific metabolic functions, as well as in vivo liver specific lipid nanoparticle mediated knockdown technology, coupled with biochemistry, cellular metabolic assays, high-resolution confocal microscopy and quantitative imaging, electron microscopy, RNASeq, proteomics and lipidomics. We believe that coupling basic knowledge in cell biology and membrane transport with physiological approaches in mice will substantially enhance our understanding of the function of intracellular trafficking components in metabolic control and has the potential to provide novel therapeutic targets and approaches for treatments of human metabolic diseases.
Endosomal regulators with a novel function in type-2 diabetes, metabolic dysfunction-associated liver disease (MASLD) and metabolic associated steatohepatitis (MASH)
Recently, the small GTPase Rab24 was found to control systemic blood glucose homeostasis via improving mitochondrial fusion/fission plasticity in the liver. Inhibition of Rab24 in HFD treated mice strongly improved liver and serum lipid and glucose metabolism by controlling mitochondrial turnover, highlighting a potential therapeutic application of Rab24 for NAFLD, thus establishing a conceptual functional connection between intracellular transport and systemic metabolic dysfunction (Seitz et al. Nat. Metab. 2019). In continuation, we are currently further investigating the role of Rab24 in mitochondria turnover, autophagy and metabolism.
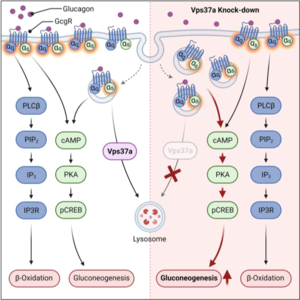
As another example we identified Vps37a, a component of the endosomal sorting complexes required for transport (ESCRT-I) to control glucagon receptor (Gcgr) trafficking and signaling in the liver by regulating the receptor intracellular localization. By shifting the receptor to endosomal membranes, we could uncouple Gcgr signaling to gluconeogenesis from β-oxidation, highlighting the importance of the spatiotemporal localization of Gcgr for its metabolic effects. Importantly, since Vps37a knockdown in animals fed a HFD led to hyperglycemia, although its overexpression reduced blood glucose levels, these data revealed a contribution of endosomal signaling to metabolic diseases that could be exploited for treatments of T2D (Sekar et al. Cell Metab. 2022). We are further exploring the role of Vps37a and other ESCRT-I members in liver physiology and pathophysiology.
Influence of metabolic nutritional cues (e.g. fasting/feeding, HFD) on the function of the endosomal and secretory membrane transport system using organelle and phospho-proteomics
The fasting/refeeding transition is a metabolic process essential for whole body physiology. During the step from fasting to feeding, hepatocytes need to turn down glucagon and speed up insulin signaling to mediate the postprandial response. This is achieved by intracellular spatial and temporal remodeling of the distribution of activated protein kinases and downstream interactors, where the endosomal system plays an essential part in this reorganization. Thus, we are investigating the response of the endocytic network during the fasting/feeding switch in the liver.
Development of an in vitro system of cryopreserved primary steatotic human hepatocytes for target & drug validation and screening
As MASLD is a serious pre-requisite for the development of MASH and is increasing worldwide without any available drugs, it is essential to find novel treatment strategies for intervention. However, since human MASLD to MASH development differs highly from rodents, a reliable human in vitro system for MASLD is required to improve our knowledge on the pathophysiology and treatability of MASLD. Therefore, we have set up a steatotic 3D in vitro system of cryoconserved primary human hepatocytes that recapitulates essential aspects of MASLD, such as steatosis, insulin resistance, mitochondrial dysfunction and inflammation and employ this system for target and drug validation studies.
The role of liver endothelial cells (LSEC) in MASLD to MASH progression
A largely unaddressed and poorly understood aspect of MASLD and T2D is to which extent liver endothelial cells (LSEC) change mechanistically during disease progression and contribute to pathogenesis. Thus, another arm our research focuses on the role of LSEC in MASLD/MASH and T2D development. In particular, we want to understand whether and to which extent the highly relevant endocytic capacity of LSEC changes during the course of the disease, which other cellular trafficking functions are affected and how this connects to the observed morphological alterations during MALSD/MASH disease progression.