Sie befinden sich hier
Inhalt
Project 1: Structural and functional heterogeneity of the AIS in projection neurons of hippocampal ensembles
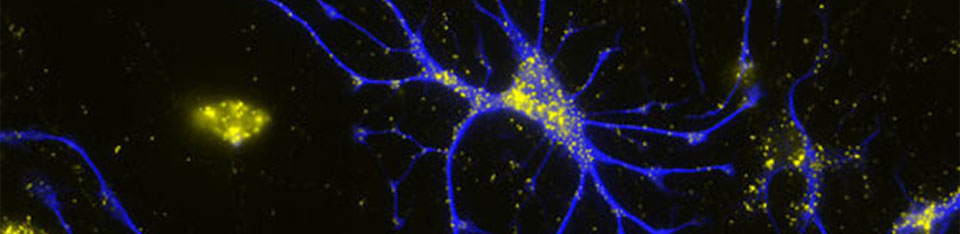
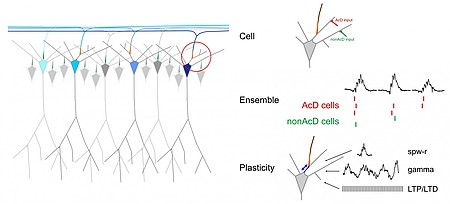
We want to investigate whether the axon origin location can modulate input-output relations of neurons and if this makes a difference in the integration of cells into functional ensembles.
Action potentials (APs) are usually initiated in the axon initial segment (AIS), a highly specialized neuronal compartment. Recent evidence suggests that location and length of the AIS is a variable and independent determinant of neuronal excitability. Recently, we have found that the AIS branches off from basal dendrites rather than from the soma in about 50% of CA1 pyramidal cells. Our preliminary data show that such axon-carrying dendrites (AcD) are privileged sites for synaptic excitation, supporting facilitated action potential generation.
This project is part (Segment A03) of the SFB1134 "Functional Ensembles".
Project 2: AnkyrinG – A molecular determinant of neuronal polarity (funded by the DFG)
Neurons are highly polarized cells that are capable of developing and maintaining two highly distinct types of cell processes: (I) the axon and (II) dendrites. The mechanisms that underly the development and maintenance of this axo-dendritic polarity are largely unknown.
Earlier in vitro studies suggest that a membrane-associated diffusion barrier, localized in the axon initial segment (AIS), contributes to the maintenance of axo-dendritic polarity. We hypothesized that the AIS-specific membrane adapter protein ankyrinG plays a major role for the function of the AIS-specific diffusion barrier thereby maintaining of neuronal polarity. This hypothesis was tested by studying neuronal polarity in mice with a cerebellum-specific knock out for ankyrinG.
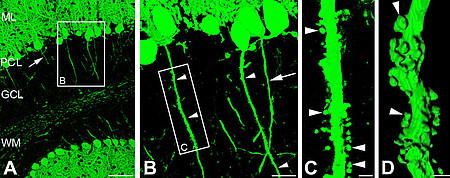
Strikingly, cerebellar axons of these animals develop a number of hallmark features normally associated only with dendrites. Most importantly, these axons are endowed with tiny protrusions closely resembling dendritic spines. Similar to their dendritic counterparts these aberrant axonal spines possess postsynaptic glutamatergic receptors and are contacted by presynaptic glutamatergic boutons as revealed by combined light- and electronmicroscopy.
We conclude that anykrinG plays a key role for maintaining appropriate axo-dendritic polarity in vivo. Currently, we are using several in vitro and in vivo paradigms to further unravel the fundamental cellular and molecular mechanisms of ankyrinG-mediated neuronal polarity.
Project 3: Neurodegenerative diseases: Tauopathies
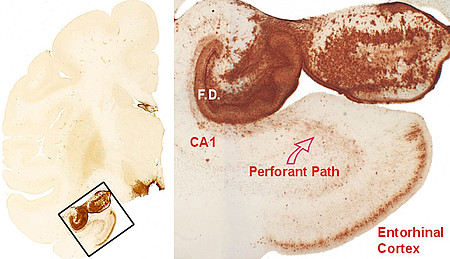
Left: Frontal hemisphere section with a conspicuous accumulation of tau patology in the medial temporal lobe. The framed box is shown at higher magnidication on the right side. Right: A dense accumulation of tau pathology is seen in the entorhinal cortex, in the white matter of the perforant pathway, and in the hippocampal formation (F.D.: fascia dentata). Adapted from Schultz et al., 2000.
The abnormal aggregation of the microtubule-associated protein tau plays a central pathogenic role in a number of neurodegenerative diseases of the human brain, together referred to as the „tauopathies“. The most important member of these diseases is Alzheimer´s disease. The development of therapeutic approaches for curing tauopathies is significantly hampered by the lack of an appropriate animal model.
Using immunhistochemistry against abnormally phosphorylated forms of tau protein we were able to document a previously unknown form of tau pathology in brains of aged non-human primates (baboons). Based on the close phylogenetic relationship between baboons and the human species, further studies on these changes may complement the work on transgenic mouse models for tau pathology.
Project 4: Sex-Dependent Hypothalamic Tauopathy (SDHT)
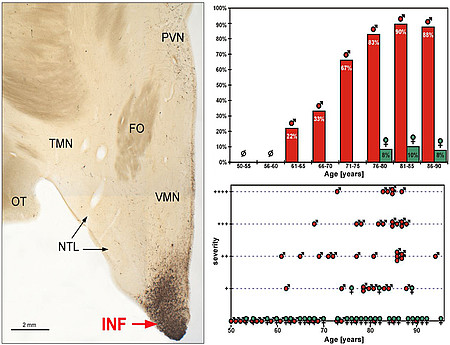
Left: Overview displaying a conspicuous accumulation of AT8 immunoreactive structures in the infundibular nucleus (INF). Note the virtual absence of tau pathology in adjacent hypothalamic areas. PVN paraventricular nucleus, TMN tuberomamillary nucleus, FO fornix, NTL lateral tuberal nucleus. Right: Statistical analysis demonstrating the striking sex difference of SDHT which preferentially affects elderly males. Adapted from Schultz et al. 1999.
A group of human neurodegenerative disorders, the so-called “tauopathies,” are characterized by abnormal phosphorylation and aggregation of the microtubule-associated protein tau in the form of intracelluar fibrillar inclusions. The most important member of these of diseases is Alzheimer´s disease. In a well-defined region of the human hypothalamus - known as the infundibular nucleus (INF) - we recently discovered a novel type of tauopathy.
Remarkably, this hypothalamic tauopathy predominantly involves elderly males, whereas women are rarely affected. Further studies indicate that these sex-dependent changes develop indepedentendly of Alzeimer´s disease. We have coined the term "sex-dependent hypothalamic taupathy" (SDHT) to refer to this unique entity of cytoskeletal pathology in the human brain. Further studies are required to pinpoint the clinical implications of SDHT.
Kontextspalte
Group Leader

Prof. Dr. Christian Schultz
Phone +49 621 383-71555
christian.schultz@medma.uni-heidelberg.de