Sie befinden sich hier
Inhalt
Most of our recent understanding about synaptic signal integration and plasticity is centered on the somatodendritic domain of neurons. The axonal domain, in contrast, has been viewed as a stable neuronal output feature only. Recent work in the field of cellular neurobiology is challenging this view. We are beginning to unravel a far more dynamic role for axonal domains in neuronal signal processing and its relation to development, function, and pathology of neurons, also in a network context. Axons, just like dendrites, contribute to the activity-dependent modulation of neuronal function in many ways.
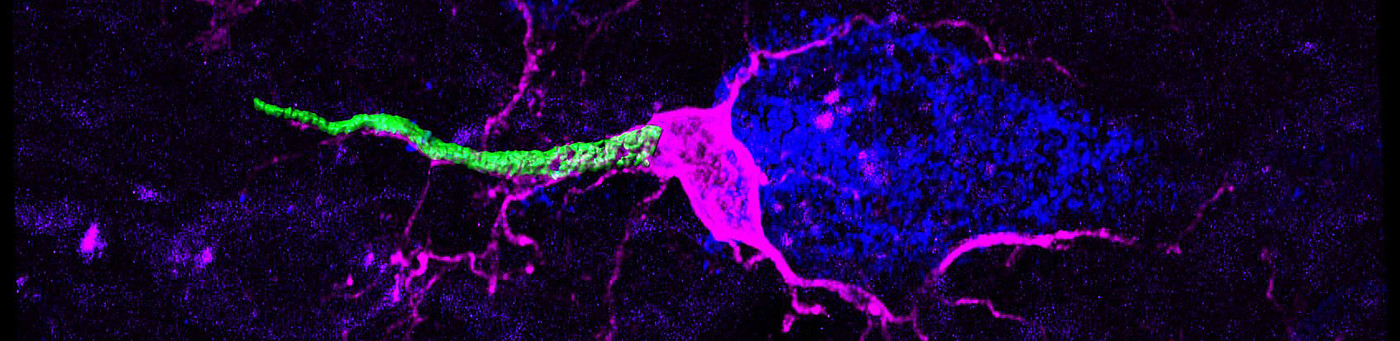
In particular, the axon initial segment (AIS), often localized to the most proximal part of the axon and responsible for action potential initiation, is emerging as a hub of dynamic signaling modulation. Our research therefore explores AIS plasticity in physiological, developmental and pathophysiological contexts. Furthermore, we look at factors contributing to axonal domain development in the late embryonic and early postnatal phase in rodents. We employ advanced histological, biochemical, microscopic and electrophysiological methods to address these topics with an interdisciplinary approach.
Project 1: Activity-dependent AIS plasticity in sensory systems
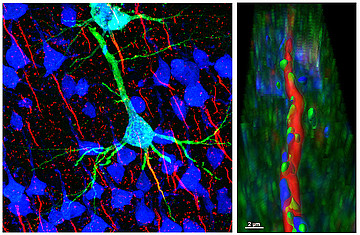
In the context of AIS plasticity, we are studying the rodent visual and somatosensory systems both during development as well as in the adult, and test how activity-dependent mechanisms drive AIS formation and maturation. Further, we employ both sensory deprivation and over-stimulation to study AIS plasticity in adult systems. Previous work from this topic includes an MD thesis by Annika Gutzmann highlighting a period of structural plasticity at the AIS in visual cortex (Gutzmann et al., 2014) and a PhD thesis by Annabelle Schlüter (Schlueter et al., 2017), showing the involvement of intra-axonal calcium stores in AIS plasticity in the visual cortex.
Current projects include MD thesis work by Nora Jamann (rapid AIS plasticity in barrel cortex in vivo), Johannes Roos (activity-dependent modulation of AIS assembly in organotypic cultures) and Felix Höfflin (Höfflin et al., 2017), who investigates morphological heterogeneity of the AIS in different neuron subpopulations. Also, in the context of adult neurogenesis, we are investigating the development and maturation of the AIS in a population of tangled cells that reside in the piriform cortex and are emerging as a new type of neurogenic cells (MD thesis Dominik Dannehl; recipient of the Faculty’s Promotionsstipendium; in collaboration with Sébastien Couillard-Després and Bruno Benedetti from the Institute for Experimental Neuroregeneration (Paracelsus Private University, Salzburg, Austria).
We recently published a review article summarizing activity-dependent axonal plasticity to highlight the latest advances in the field (Jamann*, Jordan*, Engelhardt, 2017; * equal contribution).
Project 2: The AIS in models of human disease
The AIS and its master scaffolding protein, Ankyrin-G, have been implicated in several human pathophysiologies. We are studying at three different models of human disease and injury to better understand the molecular mechanisms that contribute to these pathologies. We currently investigate Ankyrin-G isoforms and AIS maintenance in a rat model of spinal cord injury (MD thesis Dominik Dannehl). The main aim of this study is to uncover the AIS response to spinal cord injury at the level of layer 5 neurons in motor cortex and their axo-axonic innervation pattern.
Another project investigates AIS alterations in a rat model of epilepsy (MD thesis Florian Katgely). Last but not least, we address a human cognitive disease, autism spectrum disorder (ASD) in this context. MD candidate Merryn Jordan, another recipient of the Faculty’s Promotionsstipendium, is comparing two relevant animal models, a genetic (FoxP1) and an acquired (VPA) one, looking for alterations in the axonal compartment of striatal and cerebellar neurons in vivo.
Project 3: Neonatal inflammation and its impact on neuronal development
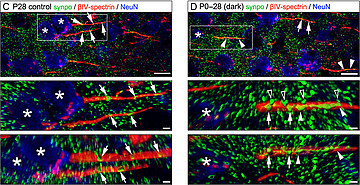
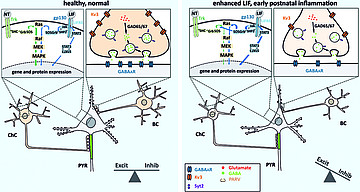
Maternal (prenatal) immune activation (MIA) and perinatal stress or inflammatory processes lead to an impaired function of interneurons in the offspring, a development discussed as being causal for the progress of mental diseases later in life. In collaboration with the Developmental Neurobiology group of Petra Wahle at Ruhr-University Bochum, we have investigated which factors play a role for normal neuronal development in the visual system (Wahle et al., 2003; Engelhardt et al., 2007) and how these factors become unbalanced under MIA conditions (Engelhardt et al., 2017, 2018). We continue this line of work, specifically investigating the development of axonal compartments under the influence of neurotrophic and cytokine signaling.
Publications
- Jamann N, Dannehl D, Lehmann N, Wagener R, Thielemann C, Schultz C, Staiger J, Kole MHP, Engelhardt M: Sensory input drives rapid homeostatic scaling of the axon initial segment in mouse barrel cortex. Nat Commun 2021, 12(1):23.
- Beutel T, Dzimiera J, Kapell J, Engelhardt M, Gass A, Schirmer L: Cortical projection neurons as a therapeutic target in multiple sclerosis. Expert Opin Ther Targets 2020, 12: 1211-1224.
- Benedetti B*, Dannehl D*, Janssen JM, Corcelli C, Couillard-Després S*, Engelhardt M*: Structural and functional maturation of rat primary motor cortex layer V neurons. Int J Mol Sci 2020, 21(17): 6101. *shared authorship
- Benedetti B*, Dannehl D*, König R, Coviello S, Kreutzer C, Zaunmair P, Jakubecova D, Weiger TM, Aigner L, Nacher J, Engelhardt M, Couillard-Després S. Functional Integration of Neuronal Precursors in the Adult Murine Piriform Cortex. Cereb Cortex 2020, 30(3): 1499-1515.
*shared authorship - Benedetti B*, Dannehl D*, König R, Coviello S, Kreutzer C, Zaunmair P, Jakubecova D, Weiger TM, Aigner L, Nacher J, Engelhardt M, Couillard-Despres S: Functional integration of neuronal precursors in the adult Murine piriform cortex. Cereb Cortex, 2019, bhz181. *shared authorship
- Schlüter A, Rossberger S, Dannehl D, Janssen JM, Vorwald S, Hanne J, Schultz C, Mauceri D, Engelhardt M: Dynamic regulation of synaptopodin and the axon initial segment in retinal ganglion cells during postnatal development. Front Cell Neurosci, 2019, 13:318
- Malek Mohammadi M, Abouissa A, Azizah I, Xie Y, Cordero J, Shirvani A, Gigina A, Engelhardt M, Trogisch FA, Geffers R, Dobreva G, Bauersachs J, Heineke J: Induction of cardiomyocyte proliferation and angiogenesis protects neonatal mice from pressure overload-associated maladaptation. JCI Insight, 2019, 23:5
- Engelhardt M*, Jamann N, Wefelmeyer W*: Small domain – large consequences: the axon initial segment as a key player in neuronal excitability. Neuroforum, 2019, 25(1). *shared authorship
- Ernst L, Darschnik S, Roos J, González-Gómez M, Beemelmans C, Beemelmans C, Engelhardt M, Meyer G, Wahle P: Fast prenatal development of the NPY neuron system in the neocortex of the European wild boar, Sus scrofa. Brain Stuct Funct 2018, 223(8)
- Rotheneichner P, Belles M, Benedetti B, König R, Dannehl D, Kreutzer C, Zaunmair P, Engelhardt M, Aigner L, Nacher J, Couillard-Despres S: Cellular plasticity in the adult murine piriform cortex: continuous maturation of dormant precursors into excitatory neurons. Cereb Cortex, 2018, 28(7)
- Engelhardt M, Hamad MIK, Jack A, Ahmed K, König J, Rennau LM, Jamann N, Räk ,A, Schönfelder S, Riedel C, Wirth MJ, Patz S, Wahle P: Interneuron synaptopathy in developing rat cortex induced by the pro-inflammatory cytokine LIF. Exp Neurol, 2018; Apr 302
- Höfflin F*, Jack A*, Riedel C, Mack-Bucher J, Roos J, Corcelli C, Schultz C, Wahle P*, Engelhardt M*: Heterogeneity of the Axon Initial Segment in Interneurons and Pyramidal Cells of Rodent Visual Cortex. Front Cell Neurosci; 2017 Nov 6. *shared authorship
- Schlüter A, del Turco D, Deller T, Gutzmann A, Schultz C, Engelhardt M: Dynamic regulation of synaptopodin in the axon initial segment during visual cortex development. Cereb Cortex, 2017, 27
- Jamann N*, Jordan M*, Engelhardt M: Activity-dependent axonal plasticity in sensory systems. Neurosci, 2017, 268. *shared authorship
- Engelhardt M, Di Cristo G, Grabert J, Patz S, Maffei L, Berardi N, Wahle P: Leukemia inhibitory factor impairs structural and neurochemical development of rat visual cortex in vivo. Mol Cell Neurosci 2017; 79
- Kübler J, Kirschner S, Hartmann L, Welzel G, Engelhardt M, Herskind C, Veldwijk MR, Schultz C, Felix M, Glatting G, Maier P, Wenz F, Brockmann MA, Giordano FA: The HIV-derived protein Vpr52-96has anti-glioma activity in vitro and in vivo. Oncotarget 2016; 7(29)
- Gutzmann A, Ergül N, Grossmann R, Schultz C, Wahle P, Engelhardt M: A period of structural plasticity at the axon initial segment in developing visual cortex. Front Neuroanat 2014; 8:1
- Thome C*, Kerry T*, Yanez A, Schultz C, Engelhardt M, Cambridge SB, Both M, Draguhn A, Beck H, Egerov AV: Axon-carrying dendrites convey privileged synaptic input in hippocampal neurons. Neuron 2014, 83(6): 1418-30. *shared authorship
Kontextspalte
Group Leader

Dr. Maren Engelhardt
Phone +49 621 383-9947
maren.engelhardt@medma.uni-heidelberg.de