Sie befinden sich hier
Inhalt
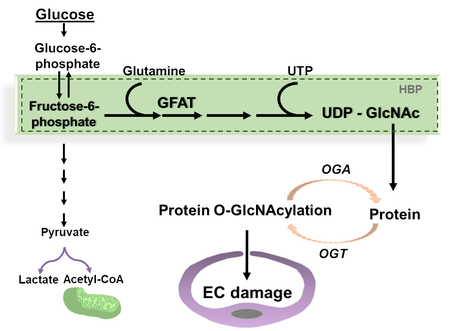
Diabetes is one of the most prevalent chronic metabolic disorders, capable of causing extensive vascular damage and organ dysfunction. A growing number of global diabetic cases has led to a concomitant rise in associated complications such as diabetic retinopathy and cardiomyopathy. Non-proliferative diabetic retinopathy is characterized by the breakdown of the neurovascular unit, initiated by endothelial damage and loss of pericytes, while proliferative diabetic retinopathy occurs due to abnormal retinal angiogenesis. Diabetic cardiomyopathy is marked by the accumulation of the extracellular matrix components, cardiac hypertrophy and cardiac dysfunction. Under physiological conditions, a small portion of cellular glucose is metabolized through the hexosamine biosynthesis pathway (HBP) to uridine diphosphate N-acetylglucosamine (UDP-GlcNAc) which is a substrate for O-GlcNAc cycle, controlled by O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA) through the attachment and detachment of N-acetylglucosamine (GlcNAc) to serine or threonine residues, respectively. Activation of the HBP, followed by increased protein O-GlcNAcylation and endothelial dysfunction have been implicated in the development of diabetic microangiopathies.
The aim of our research group is to study how the activation of the HBP in endothelial cells contributes to the pathogenesis of microangiopathies associated with metabolic disorders, and thus to provide novel intervention strategies for diabetic complications.
Models Used
In vivo:
- Streptozotocin-induced hyperglycemia-dependent mouse diabetic microangiopathy model
- Hyperglycemia-independent mouse microangiopathy model such as transgenic mouse deficient for nucleoside diphosphate kinase B in which the endothelial hexosamine pathway is highly activated
- High fat diet-induced microangiopathy model
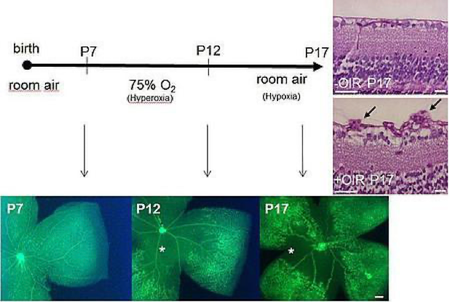
- Mouse physiological retinal angiogenesis model
- Mouse hypoxia-induced proliferative retinopathy model.
In vitro:
- Primary cell culture and established cell lines,
- Human and mouse endothelial cells, pericytes, retinal glia cells, fibroblasts, cardiomyocytes and stem cells.
Techniques
Histological analysis, immunoblotting, immunofluorescence, immunoprecipitation, proximity ligation assay, transfection with gene-specific siRNA or viral transduction of cells, ELISA, real-time quantitativePCR, RNAseq, proteomics, electroretinography, optical coherence tomography, echocardiography.
Current Major Research Focus
- Endothelial activation of the hexosamine biosynthesis pathway and retinal/cardiac dysfunction
- Cell-cell Interactions between endothelial cells in the retina and left ventricle in metabolic disorders
- Molecular mechanisms mediated by VE-cadherin, VEGF receptor 2 and angiopoietin 2 in hyperglycemia-dependent and -independent activation of the HBP
- Regulation of protein GlcNAcylation in metabolic disorders.
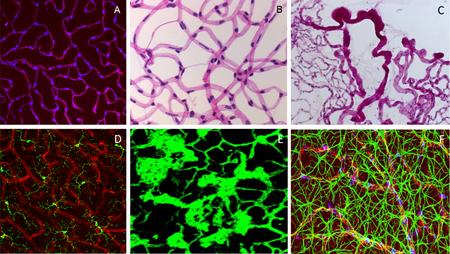
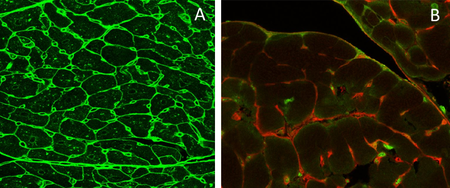
Fig. 3. A: Pericytes in retinal deep capillary layer (immunofluorescence staining, vessels labelled with lectin in red, pericytes in blue). B: Retinal digest preparation stained with PAS (endothelial cells – elongated, light blue stained nuclei; pericytes – small. Dark blur stained nuclei). C: Retinal digest preparation stained with PAS (abnormal retinal vessels). D: Retinal microglial cells (immunofluorescence staining, vessels labelled with lectin in red, microglial cells in green). E: preretinal neovascularization (immunofluorescence staining, vessels labelled with lectin in green). F: Neurovascular network in the Retina: endothelial cells (Lectin, red), pericytes (NG2, blue) and astrocytes (GFAP, green).
Project-related publications
- Chen CW, Su C, Huang CY, Huang XR, Cuili X, Chao T, Fan CH, Ting CW, Tsai YW, Yang KC, Yeh TY, Hsieh ST, Chen YJ, Feng Y, Hunter T, Chang ZF. NME3 is a gatekeeper for DRP1-dependent mitophagy in hypoxia. Nat Commun. 2024 Mar 13;15(1):2264.
- Wang Y, Eshwaran R, Beck SC, Hammes HP, Wieland T, Feng Y. Contribution of the hexosamine biosynthetic pathway in the hyperglycemia-dependent and -independent breakdown of the retinal neurovascular unit. Mol Metab. 2023 Jul;73:101736.
- Eshwaran R, Kolibabka M, Poschet G, Jainta G, Zhao D, Teuma L, Murillo K, Hammes HP, Schmidt M, Wieland T, Feng Y. Glucosamine protects against neuronal but not vascular damage in experimental diabetic retinopathy. Mol Metab. 2021 Dec;54:101333.
- Chatterjee A, Eshwaran R, Poschet G, Lomada S, Halawa M, Wilhelm K, Schmidt M, Hammes HP, Wieland T, Feng Y. Involvement of NDPK-B in Glucose Metabolism-Mediated Endothelial Damage via Activation of the Hexosamine Biosynthesis Pathway and Suppression of O-GlcNAcase Activity. Cells. 2020 Oct 19;9(10):2324.
- Shan S, Chatterjee A, Qiu Y, Hammes HP, Wieland T, Feng Y. O-GlcNAcylation of FoxO1 mediates nucleoside diphosphate kinase B deficiency induced endothelial damage. Sci Rep. 2018 Jul 12;8(1):10581.
- Qiu Y, Huang H, Chatterjee A, Teuma L, Baumann F, Hammes HP, Wieland T, Feng Y. Mediation of FoxO1 in Activated Neuroglia Deficient for Nucleoside Diphosphate Kinase B during Vascular Degeneration. Neuroglia 2018, 1(1), 271-282.
- Qiu Y, Zhao D, Butenschön VM, Bauer AT, Schneider SW, Skolnik EY, Hammes HP, Wieland T, Feng Y. Nucleoside diphosphate kinase B deficiency causes a diabetes-like vascular pathology via up-regulation of endothelial angiopoietin-2 in the retina. Acta Diabetol. 2016 Feb;53(1):81-9.
- Zhou XB, Feng YX, Sun Q, Lukowski R, Qiu Y, Spiger K, Li Z, Ruth P, Korth M, Skolnik EY, Borggrefe M, Dobrev D, Wieland T. Nucleoside diphosphate kinase B-activated intermediate conductance potassium channels are critical for neointima formation in mouse carotid arteries. Arterioscler Thromb Vasc Biol. 2015 Aug;35(8):1852-61.
- Feng Y, Gross S, Wolf NM, Butenschön VM, Qiu Y, Devraj K, Liebner S, Kroll J, Skolnik EY, Hammes HP, Wieland T. Nucleoside diphosphate kinase B regulates angiogenesis through modulation of vascular endothelial growth factor receptor type 2 and endothelial adherens junction proteins. Arterioscler Thromb Vasc Biol. 2014 Oct;34(10):2292-300.
- Hammes HP, Feng Y, Pfister F, Brownlee M. Diabetic retinopathy: targeting vasoregression. Diabetes. 2011 Jan;60(1):9-16.
Kontextspalte

Prof. Dr. Yuxi Feng
Experimental Pharmacology, European Center of Angioscience
Ludolf-Krehl-Straße 7-11
68167 Mannheim
Phone +49 621 383-71762
yuxi.feng@medma.uni-heidelberg.de